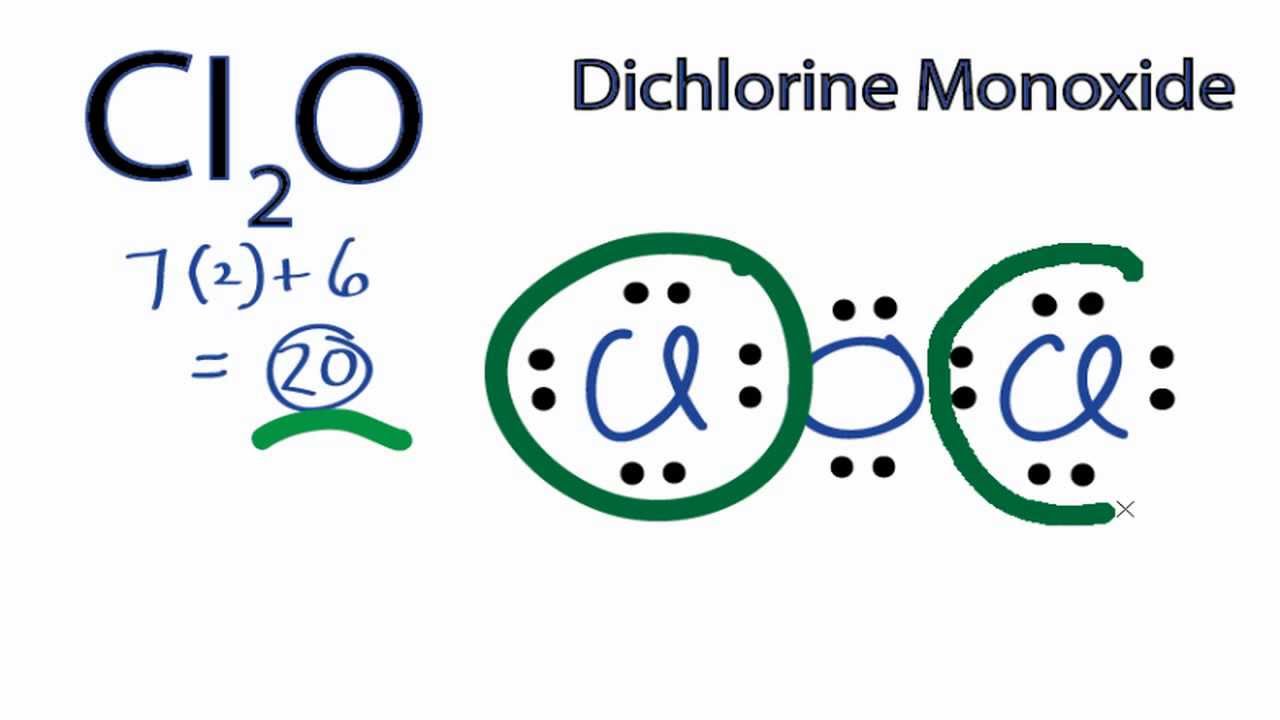
Struktur Lewis Cl2o. Determine the central atom in this molecule. What is the use of electron dot structure? Lewis structure of dichlorine monoxide (ocl2) lewis dot structure is a sketchy diagrammatical method of determining how bond formation is occurring within the participating atoms. Draw the lewis structure of hbr hydrogen bromide.
 Gambarkan Struktur Lewis Nh3 IlmuSosial.id From ilmusosial.id
Gambarkan Struktur Lewis Nh3 IlmuSosial.id From ilmusosial.id
Ikatan kovalen yang terbentuk pada senyawa ch 4, dinamakan ikatan kovalen tunggal.agar lebih memahami pembentukan ikatan kovalen tunggal , yuk pelajari ulasan berikut ini. Determine the central atom in this molecule. The cl2o can be used as is or absorbed in water to produce a hypochlorous acid solution. And oxygen, group 6 or 16, 6 valence electrons; A lewis structure is a very simplified representation of the valence shell electrons in a molecule. Compound lewis structure 1.) hcch 2.) ch3ch2oh 3.) ch3co2h 4.) o3 4 of 6 1 2 1 2
What is the lewis dot structure for cl2o?
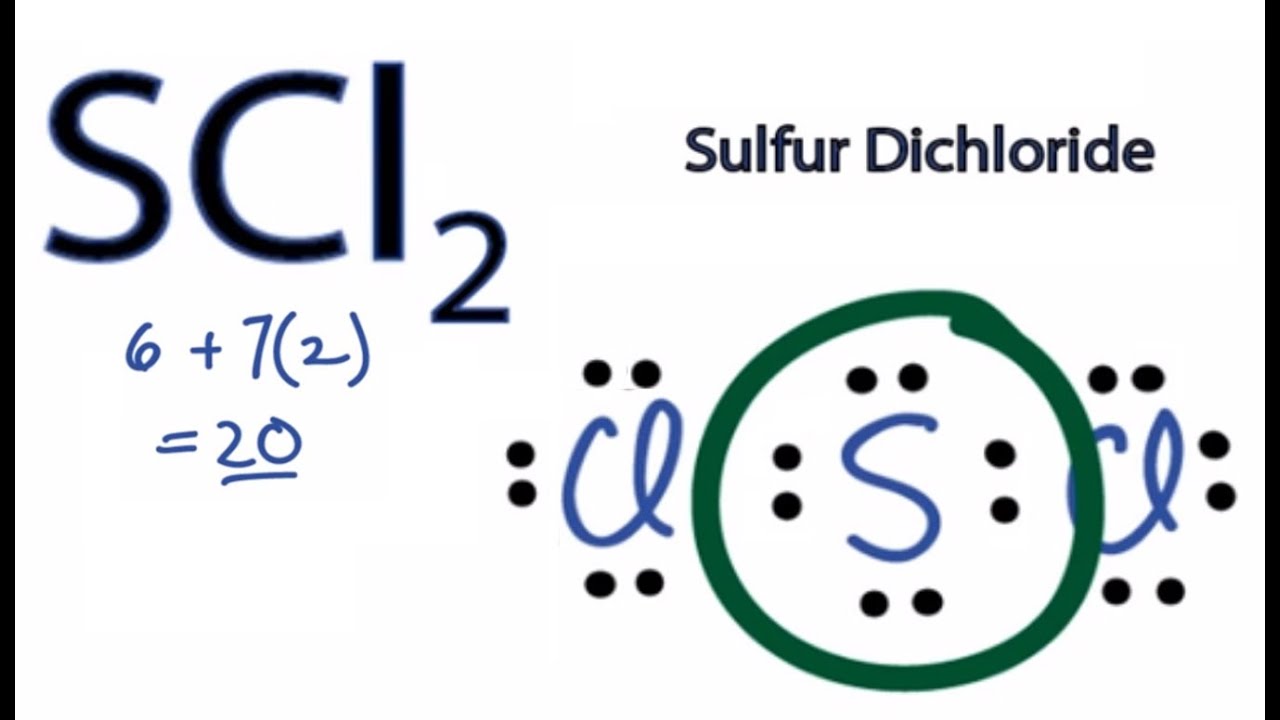
The formal charges on chlorine and oxygen are both 0. Atom 7 n memiliki konfigurasi elektron sebagai berikut 7 n: Compound lewis structure 1.) hcch 2.) ch3ch2oh 3.) ch3co2h 4.) o3 4 of 6 1 2 1 2 At room temperature it exists. This chlorine has eight valence electrons, its outer shell is full; This shows the bonding pairs, the dots are from the oxygen and the commas show the chlorine electrons.
 Source: ilmusosial.id
Source: ilmusosial.id
The oxygen will have 2 lone pairs and the chlorine atoms both ave 3 lone pairs each. We identified it from trustworthy source. Struktur lewis dalam bentuk garis. We’re being asked to determine the lewis structure and molecular geometry of cl2o2. The oxygen will have 2 lone pairs and the chlorine atoms both ave 3 lone pairs each.
 Source: primalangga.com
Source: primalangga.com
Ikatan kovalen yang terbentuk pada senyawa ch 4, dinamakan ikatan kovalen tunggal.agar lebih memahami pembentukan ikatan kovalen tunggal , yuk pelajari ulasan berikut ini. The order that the elements are written in these formulas reflects the order of attachment. High yields of cl2o are reported for the reaction of cl2, diluted with moist air, with porous soda ash. Struktur lewis dalam bentuk garis. And oxygen, group 6 or 16, 6 valence electrons;
 Source: ilmusosial.id
Source: ilmusosial.id
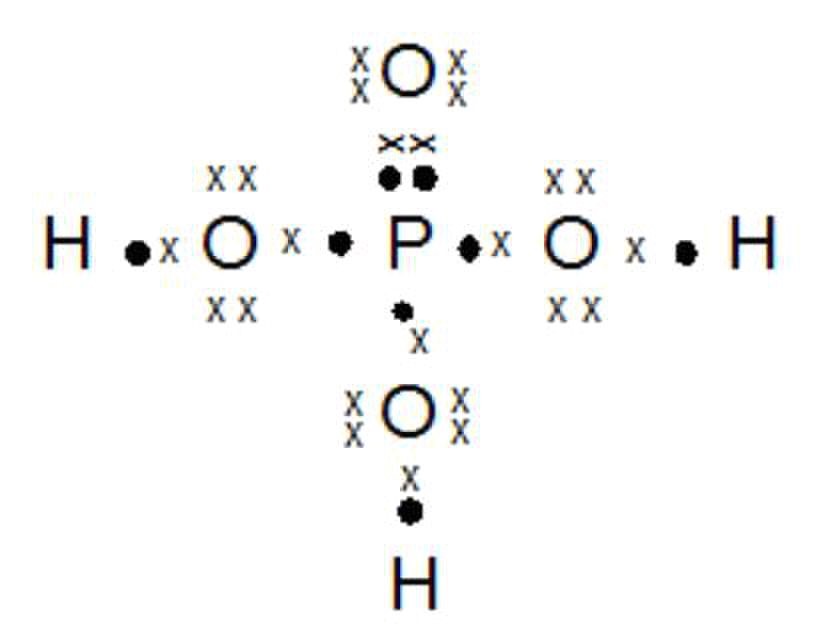
Struktur lewis untuk senyawa cl2o (ikatan kovalen tunggal), senyawa ncl3 (ikatan kovalen tunggal) dan senyawa p2o3 (ikatan kovalen koordinasi) terdapat pada lampiran. There are no charges on atoms in cl2o and we will learn how to draw the lewis structure of cl2o in this tutorial. Oxygen atom is the center atom and both chlorine atoms are located around that center oxygen atom. The formal charges on chlorine and oxygen are both 0. Chemistry 143 lewis structures dr.
 Source: ilmusosial.id
Source: ilmusosial.id
Dichlorine monoxide is an inorganic compound with the molecular formula cl 2 o. Lewis structure of dichlorine monoxide (ocl2) lewis dot structure is a sketchy diagrammatical method of determining how bond formation is occurring within the participating atoms. The vsepr or valence shell electron pair repulsion theory is a theory that explains the geometry of molecules based on repulsion between electron pairs in the valence shell. For a total of 20 valence electrons. Compound lewis structure 1.) hcch 2.) ch3ch2oh 3.) ch3co2h 4.) o3 4 of 6 1 2 1 2
 Source: brainly.co.id
Source: brainly.co.id
And the oxygen in the center also has an octet. Dichlorine monoxide is an inorganic compound with the molecular formula cl 2 o. Struktur lewis untuk senyawa cl2o (ikatan kovalen tunggal), senyawa ncl3 (ikatan kovalen tunggal) dan senyawa p2o3 (ikatan kovalen koordinasi) terdapat pada lampiran. The oxygen will have 2 lone pairs and the chlorine atoms both ave 3 lone pairs each. Oxygen is the least electronegative so it�ll go in the middle.
 Source: brainly.co.id
Source: brainly.co.id
So we’ve used all 20 valence electrons for the cl2o lewis structure. What is the lewis structure of cl2o? It is used to show how the electrons are arranged around individual atoms in a molecule. Its submitted by supervision in the best field. High yields of cl2o are reported for the reaction of cl2, diluted with moist air, with porous soda ash.
 Source: ilmusosial.id
Source: ilmusosial.id
What is the lewis dot structure of cl2o? Determine the electron pair geometry of the molecule. Dichlorine monoxide is an inorganic compound with the molecular formula cl 2 o. What is the lewis structure of dinitrogen monoxide? Struktur lewis untuk senyawa cl2o (ikatan kovalen tunggal), senyawa ncl3 (ikatan kovalen tunggal) dan senyawa p2o3 (ikatan kovalen koordinasi) terdapat pada lampiran.
 Source: brainly.co.id
Source: brainly.co.id
The structure determines how sharing of the valence electrons is taking place and whether a single, double or triple bond is forming. Its submitted by supervision in the best field. This shows the bonding pairs, the dots are from the oxygen and the commas show the chlorine electrons. Oxygen atom is the center atom and both chlorine atoms are locaated around oxygen atom. The lewis dot structure is a diagram to show the bonding between the atoms of a molecule and pairs of electrons that may exist.
 Source: avkimia.com
Source: avkimia.com
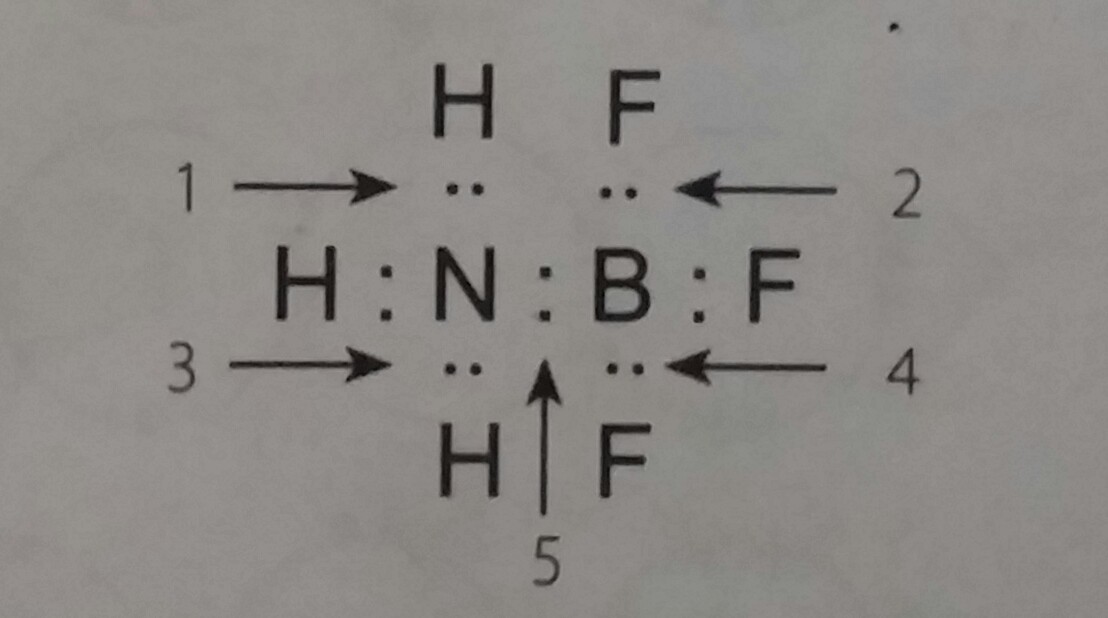
On the periodic table, chlorine is in group 7 or 7, so it has 7 valence electrons, but we have two chlorines. And the oxygen in the center also has an octet. Atom 7 n memiliki konfigurasi elektron sebagai berikut 7 n: Chemistry 143 lewis structures dr. Electrons are shown as “dots” or for bonding electrons as a line between the two atoms.
 Source: youtube.com
Source: youtube.com
There are no charges on atoms in cl2o and we will learn how to draw the lewis structure of cl2o in this tutorial. And the oxygen in the center also has an octet. The structure determines how sharing of the valence electrons is taking place and whether a single, double or triple bond is forming. In older literature it is often referred to as chlorine monoxide, which can be a source of confusion as that name now refers to the neutral species clo. A lewis structure is a very simplified representation of the valence shell electrons in a molecule.
 Source: youtube.com
Source: youtube.com
Interactive 3d chemistry animations of reaction mechanisms and 3d models of chemical structures for students studying university courses and advanced school chemistry. Caddell 2.) for each of the following compounds write the correct lewis structure in the adjacent box. Oxygen atom is the center atom and both chlorine atoms are locaated around oxygen atom. Atom 7 n memiliki konfigurasi elektron sebagai berikut 7 n: Jadi,atom n memiliki elektron valensi dengan distribusi sebagai berikut.
 Source: youtube.com
Source: youtube.com
So we’ve used all 20 valence electrons for the cl2o lewis structure. This is the cl2o lewis structure, dichlorine monoxide. And the oxygen in the center also has an octet. Penjelasan dalam sistem periodik unsur, golongan gas mulia adalah unsur yang paling stabil karena jumlah elektron valensinya adalah 8 kecuali helium 2 elektron. An unusual compound known as chlorine perchlorate dichlorine monoxide is very water soluble.
 Source: avkimia.com
Source: avkimia.com
Struktur lewis untuk senyawa cl2o (ikatan kovalen tunggal), senyawa ncl3 (ikatan kovalen tunggal) dan senyawa p2o3 (ikatan kovalen koordinasi) terdapat pada lampiran. What is the lewis structure of dinitrogen monoxide? Ikatan kimia, bentuk molekul, dan interaksi antarmolekul. At room temperature it exists. In older literature it is often referred to as chlorine monoxide, which can be a source of confusion as that name now refers to the neutral species clo.
 Source: ilmusosial.id
Source: ilmusosial.id
What is the use of electron dot structure? Dichlorine monoxide is an inorganic compound with the molecular formula cl 2 o. This is the cl2o lewis structure, dichlorine monoxide. The oxygen will have 2 lone pairs and the chlorine atoms both ave 3 lone pairs each. High yields of cl2o are reported for the reaction of cl2, diluted with moist air, with porous soda ash.
 Source: ilmusosial.id
Source: ilmusosial.id
At room temperature it exists. Struktur lewis untuk senyawa cl2o (ikatan kovalen tunggal), senyawa ncl3 (ikatan kovalen tunggal) dan senyawa p2o3 (ikatan kovalen koordinasi) terdapat pada lampiran. High yields of cl2o are reported for the reaction of cl2, diluted with moist air, with porous soda ash. So we’ve used all 20 valence electrons for the cl2o lewis structure. For a total of 20 valence electrons.
 Source: ilmusosial.id
Source: ilmusosial.id
The lewis dot structure is a diagram to show the bonding between the atoms of a molecule and pairs of electrons that may exist. Struktur lewis dalam bentuk garis. (a) oxygen is the most electronegative so it wants to have the most electrons. The number of these pairs and their role determines the bond angle due to repulsion of. There are no charges on atoms in cl2o and we will learn how to draw the lewis structure of cl2o in this tutorial.
 Source: ilmusosial.id
Source: ilmusosial.id
Clo2, dichlorine monoxide, cl2o, dichlorine hexoxide, cl2o6. Here are a number of highest rated cl2o lewis structure pictures on internet. Penjelasan dalam sistem periodik unsur, golongan gas mulia adalah unsur yang paling stabil karena jumlah elektron valensinya adalah 8 kecuali helium 2 elektron. Atom 7 n memiliki konfigurasi elektron sebagai berikut 7 n: Cl2o lewis structure how to draw the lewis structure for cl2o dichlorine monoxide.
 Source: brainly.co.id
Source: brainly.co.id
Struktur lewis untuk senyawa cl2o (ikatan kovalen tunggal), senyawa ncl3 (ikatan kovalen tunggal) dan senyawa p2o3 (ikatan kovalen koordinasi) terdapat pada lampiran. We identified it from trustworthy source. This chlorine has eight valence electrons, its outer shell is full; There are no charges on atoms in cl2o and we will learn how to draw the lewis structure of cl2o in this tutorial. We’re being asked to determine the lewis structure and molecular geometry of cl2o2.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title struktur lewis cl2o by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





